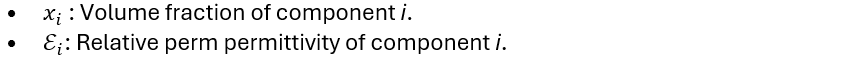What is the Debye Length
And why it matters for electrochemical biosensors
In the intricate world of electrochemical biosensors, understanding molecular interactions and their governing principles is key to unlocking high-performance sensor designs. One such fundamental concept is the Debye length. While often overlooked, the Debye length is crucial in determining the sensitivity and efficiency of charge-based detection systems. In this post, we’ll delve into what the Debye length is, how to calculate it, and why it plays a pivotal role in biosensor development.
To make this more practical, I’ll also share custom PyQt widgets you can download for calculating the ionic strength and Debye length of your solvent and explain how to determine its relative permittivity.
What is the Debye length?
The Debye length (λD) refers to the measure of a charge carriers net electrostatic effect in solution and determines how far those electrostatic effects persist. In other words, it describes how far the electric field of a charged molecule extends before it is neutralised by other ions in the solution.
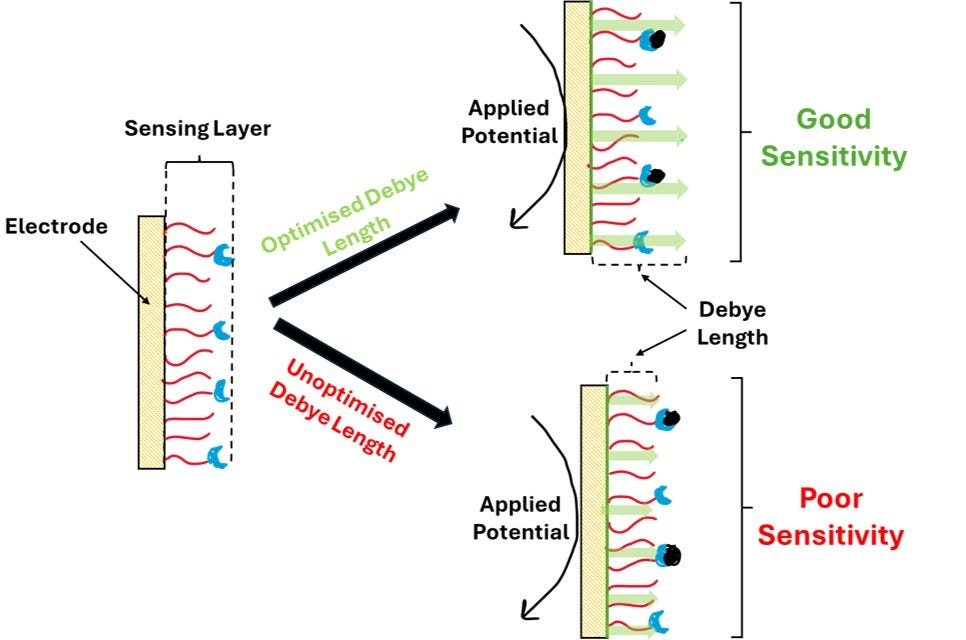
This is particularly relevant for electrochemical biosensor as when a potential is applied across an electrode surface, the local electric field strength near the surface is affected by the ionic environment. The Debye length determines how far into the solution this potential extends before it is effectively dampened by the surrounding ions. The interactions between the electrode surface potential and charged analytes are governed by this screening length.
How is the Debye length calculated?
The Debye length can be described in relation to the ionic strength (I) of your solution:
Where:
Where:
Another parameter which needs to be looked up or in some instances calculated is the relative permittivity. The of a solvent is a measure of its ability to reduce the electric field between two charges compared to a vacuum. It is critical in electrochemical systems as it affects the Debye length, ionic strength, and other parameters. Here's how you can find or determine the relative permittivity of a solvent:
1. From Literature
Standard Values: For common solvents, the relative permittivity is typically available in textbooks, chemical handbooks, or online databases. For example:
Water: ~78.5 at 25°C
Methanol: ~32.7
Acetone: ~20.7
Ethanol: ~24.5
Temperature Dependence: Relative permittivity varies with temperature, so ensure you use the value corresponding to your operating temperature.
2. Experimental Measurement
If the solvent or mixture is not well-documented, you can measure its relative permittivity experimentally using the following methods:
a. Using a Dielectric Meter
A dielectric meter measures the permittivity of liquids directly.
Place the solvent in the measurement cell and follow the manufacturer's instructions.
b. Capacitance Measurement
Set Up:
Use a capacitor with a known geometry (parallel plate capacitors are common).
Fill the capacitor with the solvent.
Measure Capacitance:
Measure the capacitance (C) of the capacitor with the solvent.
Measure the capacitance (C0) of the capacitor in a vacuum or air.
Calculate Relative Permittivity:
c. Refractive Index Correlation
For some solvents, there is a correlation between the refractive index (n) and the relative permittivity:
This approximation works for nonpolar or weakly polar solvents but is less accurate for highly polar solvents like water.
3. From Mixtures
If you’re dealing with a solvent mixture, the relative permittivity can be estimated using a weighted average based on the permittivities of the components and their volume fractions:
Where:
4. Online Tools and Databases
CRC Handbook of Chemistry and Physics: Contains permittivity values for many solvents.
Reaxys/ChemSpider: Databases with solvent properties.
Engineering Toolbox: Lists relative permittivity for common substances.
Practical Tips
Always use the permittivity value corresponding to your system's temperature and pressure.
For solvent mixtures or solutions, ensure to account for ionic strength and solute concentration, as these can influence effective permittivity.
Relevance of the Debye Length for Electrochemical Biosensing
1. Electric Field Penetration and Signal Transmission
When a potential is applied across an electrode, an electric double layer (EDL) forms at the interface between the electrode and the solution. The Debye length governs how far the influence of this potential extends into the bulk solution.
Example: In impedance-based biosensors, the Debye length dictates how deep the electric field can probe into the sensing layer or solution.