If you find this work valuable, consider supporting it by becoming a paid subscriber. For just £3.50/month, you'll unlock full access to everything Electrochemical Insights has to offer.
Paid subscribers can reach out directly to me for guidance on their research, data analysis, and experimental design!
Redox probes are fundamental to electrochemical sensing — and yet they’re often misunderstood, misused, or simply selected out of habit. If you’re working in biosensor development or supervising early-career scientists, you’ve probably encountered redox peaks that appear in the “wrong” place, with distorted shapes or reduced intensities. And when that happens, it’s tempting to blame the instrument or dismiss the result.
But in reality, these variations are windows into the surface chemistry, interface properties, and functionality of your electrochemical system.
This article is designed to give you a reference point. It covers how common redox probes behave on different electrodes, what causes deviations from expected behaviour, and how to interpret what those deviations tell you about your system.
The Role of Redox Probes in Sensor Development
Redox probes are small, electroactive molecules that undergo oxidation and reduction reactions at defined potentials. This redox activity makes them highly valuable tools in electrochemical sensor development, as they provide a direct, measurable signal that reflects the properties of the electrode–solution interface.

At their core, redox probes act as indicators of electron transfer dynamics. By monitoring their behaviour using techniques like cyclic voltammetry (CV), differential pulse voltammetry (DPV), or square wave voltammetry (SWV), researchers can gain insight into the structure, chemistry, and function of the sensor interface. These voltammetric techniques enable both qualitative and quantitative assessment of redox activity, peak potential, reversibility, and diffusion behaviour — all of which are crucial to understanding a sensor’s performance.
In Early-Stage Sensor Development
Redox probes are often the first tool used to evaluate a new electrode or validate a surface treatment. For example, when preparing a bare electrode, a researcher might run a CV using an outer-sphere redox probe like ferrocyanide or ruthenium hexamine. If the peaks are sharp, well-defined, and appear at expected potentials, the surface is likely clean and electrochemically active. If the peaks are diminished or missing, this may indicate poor surface preparation or contamination.
These early-stage tests are critical because they act as a kind of “electrochemical quality control.” A redox probe serves as a reliable reporter of how well an electrode conducts electrons — the very foundation of sensor functionality.
In Biofunctionalised Sensor Platforms
As the sensor surface becomes more complex — incorporating self-assembled monolayers (SAMs), antibodies, aptamers, enzymes, or polymers — redox probes can help track how these modifications affect electron transfer.
For instance, a properly immobilised DNA strand may partially block electron transfer from an outer-sphere probe like ferrocyanide. If a target molecule binds and causes a conformational change in the DNA structure, this may further alter access to the electrode, which is reflected in a change in the redox signal. In this way, redox probe response becomes a readout of binding events, structural shifts, or biomolecular interactions.
In enzyme-based systems, redox probes can also act as mediators, shuttling electrons between the biological recognition element and the electrode surface. Ferrocene derivatives, for example, are frequently used to mediate redox reactions in glucose biosensors and other enzyme-linked detection formats.
In Surface Characterisation and Diagnostic Testing
Beyond their role in sensing itself, redox probes are powerful diagnostic tools. Researchers often run probe-based CVs before and after each fabrication step to evaluate how the electrode surface has changed. If a monolayer has formed successfully, the peak current of an outer-sphere probe may drop or shift due to steric hindrance or charge repulsion. Conversely, if a blocking layer has failed, the redox probe may show minimal difference in current response — indicating a problem in the surface chemistry process.
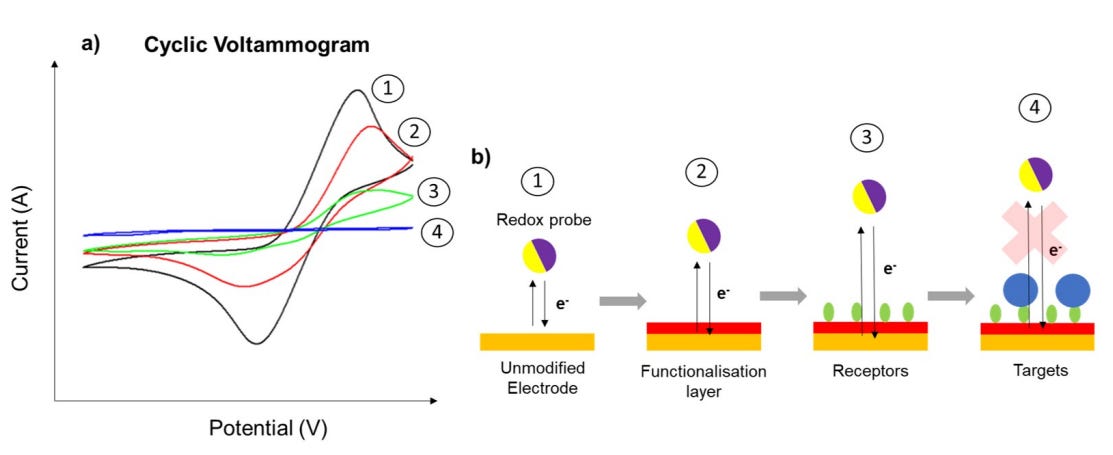
In fouling-prone environments, redox probes can be used to detect biofilm formation, protein adsorption, or degradation of protective coatings. Their sensitivity to electron transfer pathways means they effectively serve as “electrochemical reporters” for surface accessibility.
If you enjoy this content and would like to keep reading, please consider becoming a paid subscriber. For just £3.50/month get access to all Electrochemical Insights’ content .



