Electrochemical Insights is made possible entirely due to the support of its readers. If you find this work useful please consider becoming a paid subscriber. For just £3.50/month gain access to full length articles, weekly newsletters, actionable insights, downloadable tools, templates and hands-on tutorials for electrochemistry and biosensing research!
Join our growing community, where you can ask questions, share ideas, and showcase your own work. If you’re working on biosensors or electrochemical analysis and need guidance, subscribers can reach out to arrange a meeting! Whether it’s experimental design, data interpretation, or troubleshooting, I’m happy to help.
In the early 20th century, the boundaries between fundamental science and industrial research were remarkably porous. The world’s most brilliant minds weren’t just confined to universities or government-funded laboratories; they were embedded in the messy, fast-moving world of applied research—tackling problems in manufacturing, materials, and engineering that had immediate real-world consequences.
Few embodied this fusion of theory and practice better than Irving Langmuir. His name may not resonate as loudly as Einstein’s or Newton’s, but his fingerprints are everywhere—in every light bulb, in the modern science of surfaces, in vacuum technology, in plasma physics, and even in the clouds above us, where his experiments helped shape the field of weather modification.
Langmuir was not the kind of scientist who worked in an ivory tower. Instead, his lab was an old barn in Schenectady, New York, the unlikely birthplace of some of the most important discoveries in 20th-century physical chemistry. He was a Nobel laureate who spent his career inside General Electric’s Research Laboratory, an industrial setting where practical constraints became the launching pad for deep scientific exploration.
His story is not just one of genius, but of a time when fundamental and applied science were inseparable, when scientists were forced to wrestle with real-world constraints—and became better thinkers because of it.
A Mind Trained for Precision: Early Life and Education
Born in Brooklyn, New York, in 1881, Langmuir grew up in an intellectually stimulating environment. His father was an insurance executive, but both parents were meticulous record-keepers, a habit young Irving adopted early in life. This attention to detail would define his scientific career.
His first exposure to rigorous science came through his older brother, Arthur, a research chemist who encouraged young Irving to conduct home experiments. By the time he entered Columbia University, Langmuir was determined to study a field that combined chemistry, physics, and mathematics. He chose metallurgical engineering, believing it would give him the strongest foundation in all three.
After completing his undergraduate studies in 1903, he travelled to Germany to work under Walther Nernst, a leading physical chemist. There, he studied gas dissociation near hot platinum surfaces, a topic that foreshadowed his later work on surface reactions. His meticulous experiments earned him a PhD in 1906 and cemented his belief that science was not just about discovery—it was about quantifying and controlling physical phenomena.
A Scientist in Industry: The General Electric Years
Langmuir’s first job after returning to the U.S. was at Stevens Institute of Technology, but academia frustrated him. The pay was poor, and his teaching duties left little time for research. Then, in 1909, he was invited to visit the General Electric Research Laboratory in Schenectady.
The lab, which was housed in an actual barn, was an experiment in itself. It was created to be a hybrid space—separate from GE’s day-to-day operations but still dedicated to solving the company’s most pressing technical challenges. Unlike universities, where researchers had to chase funding, this lab was bankrolled by GE, which meant scientists had the freedom to pursue their curiosities—but only if those curiosities could eventually lead to something useful.
Langmuir was hooked. He joined the lab in 1909 and never left.
Fixing the Light Bulb—By Accident
At the time, GE was racing to improve the incandescent light bulb. The bulbs had several critical problems:
They blackened over time.
They burned out too quickly.
They used too much electricity.
Most of GE’s researchers believed that improving the vacuum inside the bulbs would solve the problem. Langmuir, however, took a different approach. He saw the bulb as a perfectly controlled environment for studying how gases interacted with hot surfaces—an industrial playground for fundamental research.
For three years, he experimented, measuring how different gases behaved around hot tungsten filaments. What he found completely overturned conventional wisdom:
The blackening wasn’t due to vacuum quality; it was caused by tungsten atoms evaporating and settling on the glass.
The evaporation rate could be controlled by introducing an inert gas like argon or nitrogen, preventing tungsten atoms from reaching the bulb walls.
To offset heat loss from the gas, he discovered that coiling the filament improved efficiency.
These insights led directly to a new type of light bulb—one that lasted three times longer and used half the energy.
It was a triumph of scientific thinking in an industrial setting. Langmuir wasn’t trying to improve the bulb—he was just curious about gas-solid interactions. But by following his curiosity, he accidentally revolutionized electric lighting.
The Langmuir Isotherm: Bringing Order to Surface Chemistry
Langmuir’s experience with light bulbs sparked a broader interest in how molecules interact with surfaces. While working at General Electric, he had observed that gases in a light bulb affected filament longevity and brightness, but his curiosity pushed him further. He began to wonder: how do gas molecules behave when they encounter a solid or liquid surface? This question led him to revolutionize an entire field—surface chemistry.
At the time, surface chemistry was a chaotic discipline, lacking both mathematical rigor and a clear theoretical framework. Scientists knew that molecules could adsorb (stick) to surfaces, but these interactions were described in qualitative, often inconsistent terms. No one had a solid, predictive model to describe how adsorption worked at a fundamental level.
Langmuir provided one. In 1916, he developed what is now called the Langmuir Isotherm, a simple yet powerful equation that describes how gases adsorb onto surfaces:
where:
θ represents the fraction of the surface covered by adsorbed molecules,
P is the gas pressure (or concentration in solution),
K is the adsorption coefficient, which depends on the affinity between the gas molecules and the surface.
This equation fundamentally changed the study of adsorption. It introduced the idea that gas molecules adhere to a surface in a single layer (monolayer adsorption) and that the adsorption process reaches saturation when all available sites are occupied. The Langmuir Isotherm assumed that:
All adsorption sites are identical and have the same energy.
Adsorption is reversible—molecules can stick to and detach from the surface dynamically.
No interactions occur between adsorbed molecules—each site behaves independently.
At first, this model seemed too simple, but it proved to be remarkably accurate for many real-world systems. It laid the foundation for modern surface science, influencing everything from heterogeneous catalysis (where reactions occur on solid surfaces, such as in fuel cells and catalytic converters) to sensor technology (where molecular adsorption affects signal detection in biosensors) and nanotechnology (where controlling surface properties is essential for designing functional materials).
Langmuir’s insights went beyond just gases interacting with solids. He extended his work to thin films on liquid surfaces, showing that molecules could arrange themselves in orderly monolayers—a concept that would later be used in biophysics to understand cell membranes. His findings also paved the way for later studies on colloids, detergents, and even atmospheric chemistry.
The impact of his work was so profound that it earned Langmuir the Nobel Prize in Chemistry in 1932, making him the first industrial researcher to receive the honour. This recognition was not just for the Langmuir Isotherm itself but for his broader contributions to understanding surface phenomena. Today, the Langmuir Isotherm remains a fundamental concept in chemistry, physics, and materials science, shaping how researchers think about adsorption, molecular interactions, and surface behaviour across multiple disciplines.
From Plasma Physics to Cloud Seeding
Langmuir never stopped moving between fields, and his career was defined by an insatiable curiosity that led him to make fundamental contributions in areas ranging from solid-state physics to atmospheric science. His ability to recognise universal principles across disciplines allowed him to leave an enduring impact on fields that, at first glance, seemed entirely unrelated.
In the 1920s, while studying vacuum tubes and thermionic emission at General Electric, Langmuir and his colleague Lewi Tonks stumbled upon a completely new state of matter—plasma. At the time, the scientific community only recognised three fundamental states of matter: solid, liquid, and gas. But Langmuir noticed that under certain conditions, a gas subjected to high temperatures or electrical energy would become ionised, meaning that electrons were stripped from atoms, creating a charged particle soup where electrons and ions moved freely.
This discovery led Langmuir to develop the concept of plasma, a term he coined himself, likening its behaviour to blood plasma, which also carries different particles in suspension. He found that plasma exhibited unique properties:
It could conduct electricity, unlike neutral gases.
It generated electromagnetic waves, which later became known as Langmuir waves.
It displayed self-organising behaviours, forming filaments and structures that defied the standard understanding of fluids and gases.
Plasma research quickly became essential to many areas of physics and engineering. Today, plasma physics underpins technologies such as fusion energy, neon lights, plasma TVs, and even space propulsion systems. In fact, 99% of the observable universe is composed of plasma, from the Sun and stars to interstellar clouds. Langmuir's insights into this new state of matter fundamentally shaped how we study astrophysics, controlled nuclear fusion, and space science.
Langmuir’s intellectual restlessness didn’t stop with the microscopic world of atoms and electrons. During World War II, his expertise was called upon for an entirely different problem: aircraft de-icing. Military planes operating at high altitudes often faced severe icing conditions, leading to mechanical failures and crashes. Tasked with finding a solution, Langmuir began investigating how ice forms in clouds and how it could be controlled.
This line of research led him into meteorology, where he collaborated with his assistant Vincent Schaefer. Their initial goal was to prevent ice build-up on aircraft, but their experiments uncovered something even more ground-breaking. They found that introducing small particles—nucleating agents—into clouds could induce precipitation.
Langmuir and Schaefer discovered that silver iodide had a crystal structure similar to ice, making it an effective agent for triggering the formation of ice crystals in clouds. When silver iodide was released into cold clouds, it acted as a nucleation site, encouraging supercooled water droplets to freeze and form snowflakes. The weight of these ice particles would eventually cause them to fall as rain or snow, depending on temperature.
This work launched the entire field of cloud seeding, a technique that is still in use today to enhance rainfall, mitigate droughts, and even reduce hailstorm damage. Langmuir believed cloud seeding could be used to control weather patterns on a large scale, an idea that captured the imagination of scientists and policymakers alike. However, despite early successes, cloud seeding remains controversial, with debates over its effectiveness and ethical implications continuing to this day.
A Legacy of Curiosity and Practical Innovation
Unlike many of his contemporaries, Langmuir never sought academic prestige. He remained at General Electric for his entire career, retiring in 1950 but continuing as a consultant until his death in 1957. His approach to science was refreshingly pragmatic—he worked, as he often said, “for the fun of it.”
Yet his impact is undeniable. From the Langmuir adsorption isotherm to the Langmuir probe, his name is embedded in the language of science. His pioneering work on surface chemistry influenced everything from catalysts to semiconductors. His research in plasma physics laid the foundation for fusion energy. His improvements to incandescent lighting shaped everyday life. And his investigations into cloud seeding opened new frontiers in meteorology.
Despite these achievements, Langmuir remains an unsung hero. Perhaps it is because he worked in an industrial lab rather than a university. Perhaps it is because he was more interested in solving problems than in seeking recognition. But whatever the reason, his legacy stands as a testament to the power of interdisciplinary thinking, rigorous experimentation, and boundless curiosity.
As we continue to explore the surfaces of materials, the behaviour of plasmas, and the chemistry of the atmosphere, we do so on the shoulders of Irving Langmuir—one of the greatest minds of the 20th century.
Thank you for supporting Electrochemical Insights!






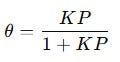



For a great read that puts Langmuir in his historical context see Cathedrals of Science